Our CPC
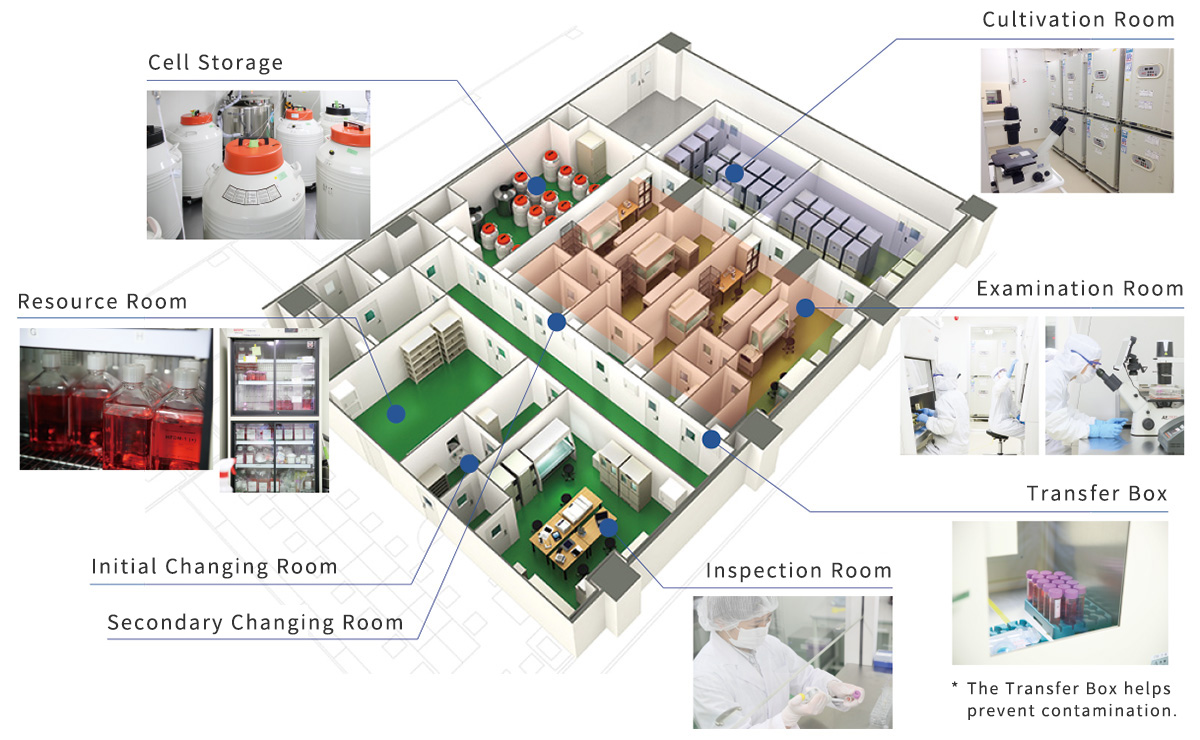
Our Cell Processing Center runs on the highest standards of GMP (Good Manufacturing Practice) outlined in the Act on the Safety of Regenerative Medicine. Health and safety is the backbone of our Quality Assurance system. Everything from air quality to cell handling practices are carefully managed to ensure that we only deliver the highest quality cells for treatment at our partner clinics.
New developments are a big part of the work that happens at our CPC. We’re always working to meet the needs of our partner clinics with new cultivation methods and new treatments.