Accessible Treatments
Large-scale cultivation, strong R&D and a careful provision system are all key parts of accessible regenerative medicine.
But with no standardized system for regenerative medicine treatments can be expensive and hard to come by. At CellBank we're working to make accessible regenerative medicine a reality. Our goal is to make regenerative medicine streamlined, safe and affordable for everyone.
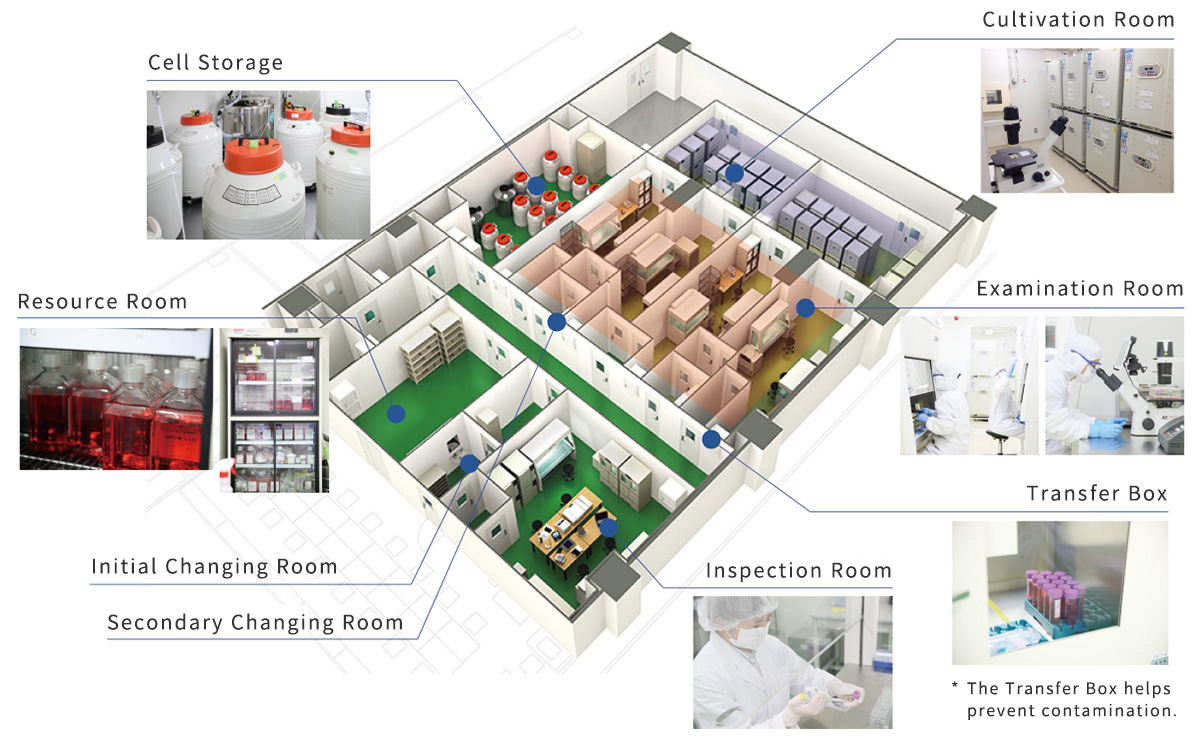
Regenerative treatments are one of the biggest breakthroughs in modern medicine. A high level of technical expertise, specialized equipment, a strong management system and highly trained specialists are all essential for quality regenerative medicine. But it can be hard for most clinics to implement highly specialized treatments. Because of this regenerative medicine has only been available to a small number of patients at a few highly specialized facilities.
Regenerative treatments are one of the biggest breakthroughs in modern medicine. A high level of technical expertise, specialized equipment, a strong management system and highly trained specialists are all essential for quality regenerative medicine. But it can be hard for most clinics to implement highly specialized treatments. Because of this regenerative medicine has only been available to a small number of patients at a few highly specialized facilities.
CellBank is working to revive regenerative medicine as a treatment option available to anyone by providing total support for the implementation of regenerative medicine at other medical facilities through our unique knowledge and experience. In 2020, 14 medical facilities began offering regenerative medical treatments in collaboration with CellBank.
We provide the knowledge and experience in introducing regenerative medicine we possess to a long list of medical institutions, so as to make regenerative medicine accessible to everyone who wants it.
Each "thank you" from a patient makes our work here at CellBank worth it. That’s why we want to make regenerative medicine accessible for everyone.